L1 /
3
CH1012

1 COMPONENT SYSTEMS
II

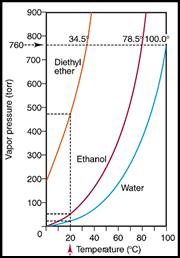
Vapour
Pressure increases with
T.
TF = freezing
point
TB = boiling
point
(V.P. = external pressure)
(V.P. = external pressure)

Cairns
(0m,760
torr)
= 100oC
= 100oC
Bartle Frere (1615m,613 torr)
= 94oC

liquid
phase
boundary
gas
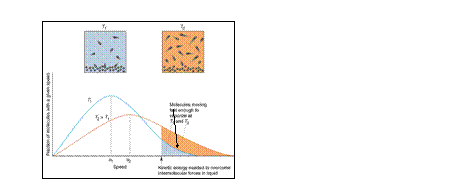
intermolecular
forces alter
b.p.
b.p.
Fraction of molecules with
sufficient Ek to escape liquid
sufficient Ek to escape liquid