L1 /
8
CH1012

CLAUSIUS-CLAPEYRON EQN.

HOW
TO OBTAIN DHvap
The
relationship between the V.P. and T
can be more readily understood by varying T, measuring p and then
plotting ln p vs 1/T.
can be more readily understood by varying T, measuring p and then
plotting ln p vs 1/T.
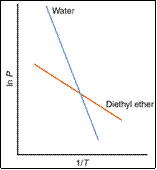
Slope = -DHvap/R
eg. H2O(l) ® H2O(g) DHvap= 40.7
kJ/
mol
mol
ln(p2/p1) =
-DHvap/R x (1/T2 -1/T1)
(cf. Arrenhius equation P597)
Clausius-Clapeyron
Eqn.
ln p = -DHvap/RT + constant