†††††††††††††††††††††††††††††††††††††††† L1 / ††
12
CH1012

BP. vs Vap.
COMPOSITION I

DGsoln†= DHsoln†- TDSsoln
Gsoln= Gibbs
Energy†† Hsoln= Enthalpy† Ssoln= Entropy
1) DGsoln†= +ve
immiscible liquids wonít mix
2) DGsoln†= -ve miscible liquids
(i)†† DHsoln† = 0
†††† pA†= poAxA,††††† pB†= poBxB
- ideal
solution behaviour
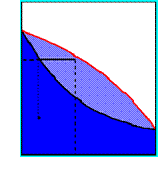
0††††††††††††† xA†††††††††† 1
T
a1

Boiling point curve

Vapour composition
curve
(here A is more volatile than B)

V.P. above mixture of
A
mole-fraction of A
V.P. pure liquid A
Temperature Composition
Diagram (p=1atm)
Vapour
Liquid

condense
heat liquid
Raoultís Law