L1 /
7
CH1012

KINETIC THEORY

There
are a range of individual energies of gas molecules
in any sample of gas.
As
the temperature rises the average kinetic
energy of the gas molecules increases.
energy of the gas molecules increases.
Kinetic energy = ½mu
2 (u
= molecular speed)
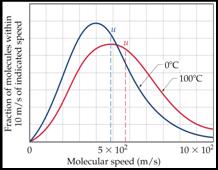
The
populations of gas
molecules with
velocity u vary as
shown with temperature.
molecules with
velocity u vary as
shown with temperature.
As
a result the kinetic
energy varies in a
similar fashion with
temperature.
energy varies in a
similar fashion with
temperature.