L1 /
9
CH1012



T drops
Ek drops
Ep(g)
Ek drops
Ep(g)


DHvap
DHfus
PHASE EQUILIBRIA

Phase Equilibria
T constant
Ek constant
Ep(l) < Ep(g)
Ek constant
Ep(l) < Ep(g)
T drops
Ek drops
Ep(l)
Ek drops
Ep(l)
T drops
Ek drops
Ep(s)
Ek drops
Ep(s)

T constant
Ek constant
Ep(s) < Ep(l)
Ek constant
Ep(s) < Ep(l)
Phases
Equilibria
Ep is the intermolecular energy - when
H-bonds form this decreases Ep
H-bonds form this decreases Ep
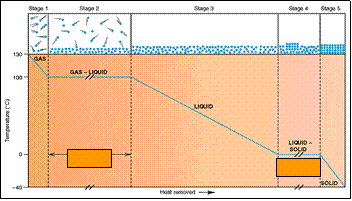
Heating - Cooling Curve (H2O)
Removal of heat from a gaseous system.
Removal of heat from a gaseous system.
normal b.p.
pvap= 1atm
pvap= 1atm
normal m.p.
pvap(s)= pvap(l)
ptotal = 1atm
pvap(s)= pvap(l)
ptotal = 1atm
T drops
Ek drops
Ep(g)
Ek drops
Ep(g)