L1 /
5
CH1012

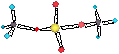
VAN DER WAALS FORCES II

3) Dispersion forces
- fluctuating,
instantaneous dipoles
- fluctuating,
instantaneous dipoles
- exist
between all
molecules including
non-polar molecules.
molecules including
non-polar molecules.
- magnitude increases with M and the number
of possible intermolecular contacts (shape)
eg. b.p. n-pentane > b.p. neopentane
CH3(CH2)3CH3 (CH3)4C
of possible intermolecular contacts (shape)
eg. b.p. n-pentane > b.p. neopentane
CH3(CH2)3CH3 (CH3)4C