L1 /
3
CH1012

METAL ALLOYS I

Alloy = a material with metallic
properties resulting from the
combination of ³ 2 elements.
properties resulting from the
combination of ³ 2 elements.
Gold 24 carat
100%
14 carat 58% alloy with Ag,Cu
14 carat 58% alloy with Ag,Cu
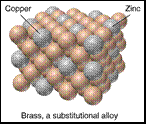
Brass 67% Cu 33% Zn alloy
1) Solution alloys - homogenous mixtures,
components dispersed randomly, uniformly.
components dispersed randomly, uniformly.
(a) Substitutional alloy - atoms of the minor
component substitute for atoms in the bulk
structure. Atoms of similar size & chemical bonding tendecies. eg. brass Cu(1.28) / Zn(1.33)
component substitute for atoms in the bulk
structure. Atoms of similar size & chemical bonding tendecies. eg. brass Cu(1.28) / Zn(1.33)
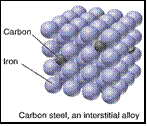
(b) Interstitial alloy - one atom type is much smaller (typ. main group) and fits into the interstices of the bulk structure. 0.02%C
eg. Fe (1.24) / C(0.71 Å)
eg. Fe (1.24) / C(0.71 Å)
Low