L1 /
7
CH1012

ELECTROLYSIS

• For very
active metals
there are no appropriate
chemicals available to
carry out the reduction
compound ® metal
\ electrolysis is used
there are no appropriate
chemicals available to
carry out the reduction
compound ® metal
\ electrolysis is used
• Electrolytic cell:
electrical energy from an
external source drives a
non-spontaneous reaction
Ecell -ve, DG +ve
electrical energy from an
external source drives a
non-spontaneous reaction
Ecell -ve, DG +ve
• the opposite of a
Voltaic
cell.
cell.
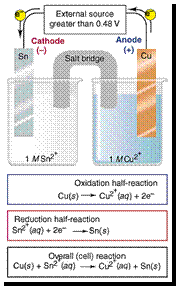
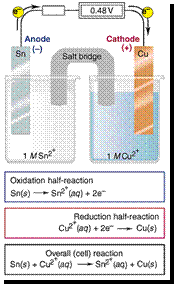
Eocell = Eocathode - Eoanode
= -0.14 - 0.34 = -0.48 V
= -0.14 - 0.34 = -0.48 V
VOLTAIC
Eocell = Eocathode - Eoanode
= 0.34 - -0.14 = 0.48 V
= 0.34 - -0.14 = 0.48 V