L1 /
13
CH1012

IRON & STEEL

To produce
1 kg Fe:
1.7 kg hematite
0.5 kg coke
0.25 kg limestone 2.0 kg air
0.25 kg limestone 2.0 kg air
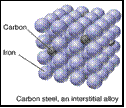
• Steel is an alloy, a material
with metallic properties resulting
from the combination of ³ 2 elements.
with metallic properties resulting
from the combination of ³ 2 elements.
carbon steel 1.0% C this is obtained
in a basic oxygen furnace where oxygen burns off the residual impurities in the pig iron
eg. Sabatier knives - soft,
v. sharp, rust
• stainless
steel 0.5%
C, Mn, Cr, Ni
eg.
Opinel knives - hard, don’t rust