L1 /
6
CH1012

OXIDATION STATE
RULES III

e.g. H - F
H + I F - I
Generally
:
F always has an oxidation state of - I
F always has an oxidation state of - I
O normally has an oxidation state of - II
H has an oxidation state of + I
THE
SUM OF OXIDATION NUMBERS IN A FORMULA MUST EQUAL THE
CHARGE WRITTEN FOR THE FORMULA
e.g OH- O - II H + I sum = - I
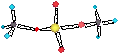
ClO4- O - II Cl +VII sum = - I (4x-2+7)